Perhaps one of the real breakthroughs in the history of dyes came in 1856 when a teenager who was experimenting at his makeshift laboratory in home made a certain discovery that acted as a sort of launching pad for the modern chemicals industry.

William Perkin
an 18-year-old student was working on chemical synthesis of natural
products. In a classic case of serendipity, the young William Perkin
chanced upon his now famous 'Aniline
Mauve' dye while he was
attempting to synthesize quinine, the only cure for malaria. Perkin
named his color Mauveine,
after the French name of non-fast colour which was made of natural
dyes. So "Mauve"
(a basic dye) was the first synthetic dye stuff. Mauve was a derivative
of coal tar. It was the first mass-produced dye, that was commercially
available and the idea was born that a color could be made in the
factory. It was indeed a revolution
Mechanism of dyes
The color of a Dye is defined by he presence of a substance
called Chromophore
in the dyes. By definition dyes are basically aromatic compounds. Their
structures have aryl rings that has delocalised electron systems. These
structures are said to be responsible for the absorption of
electromagnetic radiation that has varying wavelengths, based upon the
energy of the electron clouds.
It is actually because of this reason that chromophores do not make dyes coloured. Rather it makes the dyes proficient in their ability to absorb radiation. Chromophores acts by making energy changes in the delocalised electron cloud of the dye. This alteration invariably results in the compound absorbing radiation within the visible range of colours and not outside it. Human eyes detects this absorption, and responds to the colours.
Another possibility is that if the electrons are removed from the electron cloud, it may result in loss of colour. Removing electrons may cause the rest of the electrons to revert to the local orbits. A very good example is the Schiff's reagent. As sulphurous acid reacts with pararosanilin, what happens is that a sulphonic group attaches itself to the compound's central carbon atom. This hampers the conjugated double bond system of the quinoid ring, and causes the electrons to become localised. As a consequence the ring ceases to be a chromophore. As a result, the dye becomes colourless.
To conclude chromophores are the atomic configurations which has delocalised electrons. Generally they are represented as carbon, nitrogen, oxygen and sulphur. They can have alternate single and double bonds.
Mechanism of color changing of the dyes
Color of the dye are altered by the Colour modifiers like methyl or ethyl groups can actually alter the colour of dyes. They do so by altering the energy in the delocalised electrons. It has been found that by addition of a particular modifier there is a progressive alteration of colour. An example can be given for methyl violet series.
Color modifiers effects the colors as followong:
Step A: When no methylgroup is added the original dye Pararosanil as it is called is red in colour.
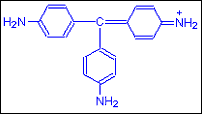
Step B: As Four Methyl groups are added the reddish purple dye Methyl Violet is got
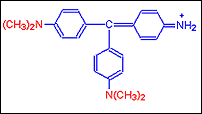
Step C: With the addition of more groups a purple blue dye Crystal Violet is obtained. It has in it six such groups.
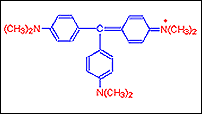
Step D: Further addition of a seventh methyl group the dye that is got is called Methyl green.
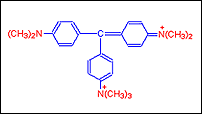
Classification of Dyes
Dyes can be classified in many ways. It should be noted that each class of dye has a very unique chemistry, structure and particular way of bonding. While some dyes can react chemically with the substrates forming strong bonds in the process, others can be held by physical forces. Some of the prominent ways of classification is given hereunder.
It is actually because of this reason that chromophores do not make dyes coloured. Rather it makes the dyes proficient in their ability to absorb radiation. Chromophores acts by making energy changes in the delocalised electron cloud of the dye. This alteration invariably results in the compound absorbing radiation within the visible range of colours and not outside it. Human eyes detects this absorption, and responds to the colours.
Another possibility is that if the electrons are removed from the electron cloud, it may result in loss of colour. Removing electrons may cause the rest of the electrons to revert to the local orbits. A very good example is the Schiff's reagent. As sulphurous acid reacts with pararosanilin, what happens is that a sulphonic group attaches itself to the compound's central carbon atom. This hampers the conjugated double bond system of the quinoid ring, and causes the electrons to become localised. As a consequence the ring ceases to be a chromophore. As a result, the dye becomes colourless.
To conclude chromophores are the atomic configurations which has delocalised electrons. Generally they are represented as carbon, nitrogen, oxygen and sulphur. They can have alternate single and double bonds.
Mechanism of color changing of the dyes
Color of the dye are altered by the Colour modifiers like methyl or ethyl groups can actually alter the colour of dyes. They do so by altering the energy in the delocalised electrons. It has been found that by addition of a particular modifier there is a progressive alteration of colour. An example can be given for methyl violet series.
Color modifiers effects the colors as followong:
Step A: When no methylgroup is added the original dye Pararosanil as it is called is red in colour.

Step B: As Four Methyl groups are added the reddish purple dye Methyl Violet is got

Step C: With the addition of more groups a purple blue dye Crystal Violet is obtained. It has in it six such groups.

Step D: Further addition of a seventh methyl group the dye that is got is called Methyl green.

Classification of Dyes
Dyes can be classified in many ways. It should be noted that each class of dye has a very unique chemistry, structure and particular way of bonding. While some dyes can react chemically with the substrates forming strong bonds in the process, others can be held by physical forces. Some of the prominent ways of classification is given hereunder.
- Organic/Inorganic
- Natural/Synthetic
- By area and method of application
- Chemical classification- Based on the nature of their respective chromophores.
- By nature of the Electronic Excitation(i.e, energy transfer colorants, absorption colorants and fluorescent colorants).
- According to the dyeing
methods
- Anionic(for Protein fibre)
- Direct(Cellulose)
- Disperse(Polyamide fibres)
- However the most popular classification is the one that is advocated by the US International Trade Commission. This system classifies dyes into 12 types.
| Group | Application |
| Direct | Cotton, cellulosic and blended fibres |
| Vat dyes | Cotton, cellulosic and blended fibres |
| Sulphur | Cotton, cellulosic fibre |
| Organic pigments | Cotton, cellulosic, blended fabric, paper |
| Reactive | Cellulosic fibre and fabric |
| Disperse dyes | Synthetic fibres |
| Acid Dyes | Wool, silk, paper, synthetic fibres, leather |
| Azoic | Printing Inks and Pigments |
| Basic | Silk, wool, cotton |

